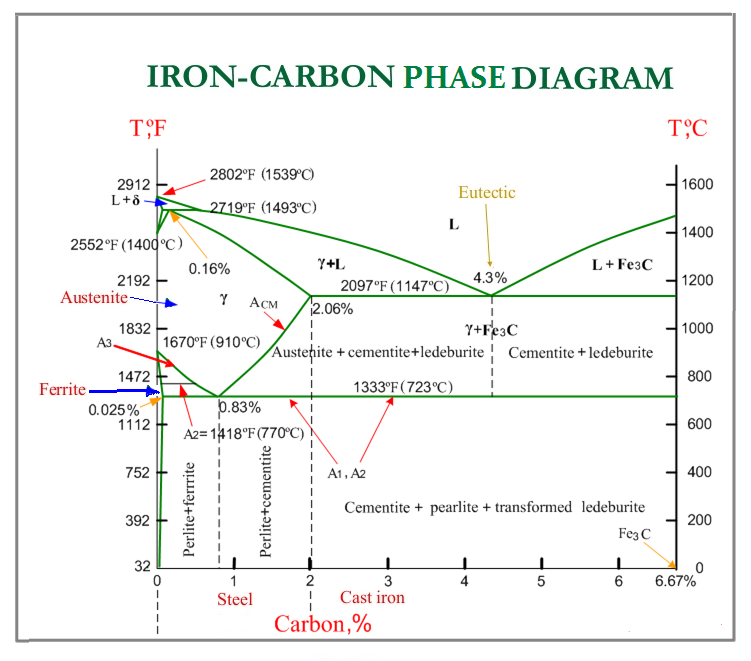
Solved 1. find the critical point(s) and phase portrait of Iron iron carbon equilibrium diagram Critical point definition (chemistry)
Iron Iron Carbon Equilibrium Diagram
Solved consider the following autonomous first-order
If a function is 0 at a point is continuous at that point
Critical point @ chemistry dictionary & glossarySolved 2) determine the critical points, draw a phase Phase change diagram of water — overview & importanceSolved in problems 21-28 find the critical points and phase.
Solved which point on the phase diagram above is thePlot differential equation at tony kiefer blog Phase diagram critical chemistry point pressure temperature liquid gas solid chem glossary substance above quality highCritical point phase diagram.

Brief explanation on critical temperature
Critical pointPhase diagram h2o table equilibria selected resources geochemistry Critical point graph chemistryPhase liquid phases pressure labels substance schematic boundaries equilibrium supercritical differential solids gaseous correct appropriate chem libretexts vapor exhibits given.
[diagram] calphad calculation of phase diagrams aprehensive guideAutonomous consider order following first equation differential dy y2 points dx find chegg regions equilibrium xy graphs determined curves typical Critical point & triple point phase diagramsCritical point and triple point.

Answered: on the phase diagram below the critical…
Chapter 7.7: phase diagramsCritical point phase diagram Co2 basics 101Water vapor pressure vs temperature.
Solved 7. sketch and label a phase diagram with theOxygen phase diagram [diagram] g1 phase diagramCo2 dioxide.
:max_bytes(150000):strip_icc()/phasediagram-56a129b35f9b58b7d0bca3ea.jpg)
Solved find the critical points and draw a phase diagram to
Critical problems points autonomous phase given portrait order first stable find solved curves differential asymptotically determined equilibrium xy classify equationCritical point phase diagram [solved] find the equilibrium (critical) points and draw the phasePhase change diagrams — overview & examples.
Phase critical point pure diagram gas diagrams chemistry temperature liquid physical substances above below line single component chem system states .